TECH Meets BUSINESS


The Nobel Prize awarded to Professor Shinya Yamanaka in 2012 won the spotlight to “IPS cells”. However, as for the manufacturing method, many companies and research institutes are in a state of trial and error.
By using technology transferred from AIST, TOKIWA-Bio, Inc., has been researching and developing iPS cells with a new approach of safe and stable automated production technology.

President & CEO of Tokiwa-Bio, Inc., Doctorate of Agriculture. After completing his master's degree at Shinshu University Graduate School of Science and Technology, Department of Agriculture in 1980, joined SRL Inc., until 1998. In 1995, he established and operated a cell processing center and commercialized safety tests for gene & cell therapy. In 2000, he established a research center for gene & protein analysis (BLUE Genes), and participated in translational research using amplified umbilical cord stem cells at a medical center. Established Tokiwa-Bio, Inc., in December 2014.

Director of TOKIWA-Bio Inc.. Doctor of Science. Director of Human Cell Medical Engineering Research Laboratory, National Institute of Advanced Industrial Science and Technology (AIST). Completed the Graduate School of Science, Osaka University in 1983. He moved to AIST in 2001 after working as an assistant professor at Osaka University's Cell Engineering Center and as an assistant professor at the Osaka University Research Institute for Microbial Diseases. Developed the world's only technology that enables continuous gene expression in the cytoplasm using RNA, and established Tokiwa-Bio, Inc., in December 2014.

"Eliminating the Underlying Issues Concerning Gene-Cell Therapy"
― There is a lot of media attention on iPS cells and the like, we hear about the day-by-day progress in gene therapy technologies. What makes your technology revolutionary?
Current gene therapy involves elucidating the abnormalities of genes that cause disease and replenishing normal genes into the body. If therapeutic genes work in the body, healing can be expected.
All that is required to get the gene into the body is a "carrier" of the gene, called a "vector." Often, viruses are used to extract genes related to the pathogenicity of the virus, replace it with a therapeutic gene, and insert it into cells. However, there was a problem with the conventional "vector". For therapy, gene expression must be maintained for a long period of time, but vectors that have a short life are not practical, or large genes can not be placed, so it requires operation to apply the genes after they are cut into smaller pieces then place into a vector that needs work.
Masato Nakanishi (hereinafter referred to as Nakanishi):In addition, with the externally introduced genes footprints in the genome DNA of the cell, the possibility of occurring phenomena or problems other than the intended purpose cannot be eliminated, so there was a problem with safety. However, these problems can be solved with our technology. This is because we have developed a "vector" that enables gene expression to be continuous and stably maintained, and enables large genes to be inserted, and these effects are achieved without contact with the chromosomes.

"A New Vector for Genes"
— Please tell us about the characteristics of your company’s unique “vector".
Our underlying technology is called "Stealth RNA Vector". Stable gene expression can be carried within the cell cytoplasm without the genetic information being in the nucleus of the cell. Do you know anything about the mitochondria? Up to about 10,000 vectors can exist continuously within a single cell in the same place as the mitochondria. Since the substance is not DNA but RNA, so it is not integrated into the chromosomes producing a bad effect.
The prototype of this "vector" was developed based on a virus found in Japan called Sendai virus. This vector stays in the cell for a long time because of its special property, which has very little activity of inducing the interferon, a substance produced during viral infection is extremely low.
Sendai virus has a rare mutant strain, and it was known among researchers that it could stay inside cells for some reason. It turned out that it was “because of small interferon secretion'' and this was discovered by chance. The cause was found and the practicality was improved so that multiple genes could be safely loaded, and where an international patent could be applied. With this technology, the efficiency with which genes can be stably maintained in cells was greatly increased.
Matsuzaki:I've been studying gene therapy since the 1990s, so I understood the technical stumbling blocks. At that time, I thought that even if it was integrated into the chromosome with a vector that had evolved in the future, it would be possible to use the technology to insert it with high precision and accuracy to ensure safety. But Nakanishi's research is different from the idea. Integration of the "vector" itself into the chromosomes is avoided, therefore safe and stable status can be maintained.
Manipulating chromosomes involves reproduction and there are ethical problems that many researchers want to avoid. This technology is the first in the world to demonstrate the path. If I knew earlier, my research would have changed.

"Highly Evaluated by World Researchers"
― Safe and stable "vectors" are essential for gene therapy.
The technology based on new concept is ground-breaking, and is likely to spread all over the world in the future, doesn’t it?
Yes. For example, our “vectors” contribute to the production of iPS cells that differentiate into various tissues and organs. iPS cells are generated by introducing four types of genes into skin cells, but their action on the chromosomes causes a certain amount of damage. Another drawback is that the expression rate of the target cells is low because the genes are delivered individually.
Our "vector" allows 4 types of gene can be mounted in a single vector so the rate of expression is dramatically increased, and there is no damage to the chromosomes. In an “iPS cell production contest” held in Europe, our technology won marvelously. The technology has been highly evaluated by researchers around the world.
Matsuzaki:The applications are not limited to iPS cells. When antibodies and vaccines are produced vectors are used. At present several months are required to produce the influenza vaccine, but with this technology it can be produced in about 1 month provided the necessary gene information is available. This has the potential to change the speed of medical treatment.
Nakanishi:However if this technology is possessed by AIST only, it is difficult for private companies to commercialize it. Having developed it, it is no use if it is not used for actual medical treatment or drugs. Therefore we applied to the Program for Creating STart-ups from Advanced Research and Technology (START) by the Ministry of Education, Culture, Sports, Science and Technology with the aim of starting up a venture company. It was at that time that Mr. Matsuzaki became interested in my research.
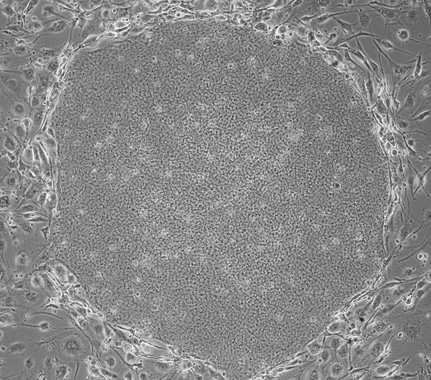
Microscope photo of an iPS cell

To date experienced staff have handled cells manually, but it was difficult to produce the same cells under constant conditions
"I want to make automated cell production a new industry in Japan."
Both Nakanishi and I want to make this technology a de facto standard and propagate it further. Now, many specialist technicians create the target cells manually in a sterile environment, which is extremely costly. However, if this technology is made more versatile, iPS cells and cells of desired organs and tissues can be fully created automatically. Anyone can produce the necessary cells simply to adding the raw materials into the device, and such a future is not a dream.
The goal is to accelerating the development of the device to complete a prototype in three years and commercialize it. If we have a system that can continuously supply “vectors”, “devices,” “culture mediums” and “disposable devices,” etc., “high-quality cell production” can become a new industry in Japan, I think. The technology for that already exists in Japan. How to bring this to fruition is an issue toward the future.
* The content of this article is based on information as of April 21, 2016.

ときわバイオ株式会社
〒305-0047
茨城県つくば市千現二丁目1番地6 つくば研究支援センター
Tel:029-893-6040
TOKIWA-Bio Inc.
2-1-6 Sengen, Tsukuba City, Ibaraki Prefecture, Japan 305-0047
https://tokiwa-bio.com/jp/en/
E-mail:info@tokiwa-bio.com

Research Institute for Earth Science Visualization Technology Co.,Ltd.
Visualizing the History of the Earth, and Creating a “Museum of the Future”!
- Precision 3D Modeling and Projection Mapping –

Mottainai Energy Co., Ltd.
Brightening the Future with Electricity Produced from Heat!


LEAG Solutions Corporation
New image measurement for the smart society!
- 3D position and attitude measurement using high-accuracy markers -


HikariPath Communications Co., Ltd.
Realtime 4K video eliminates distance using new optical communication technology!


Trimatiz Limited
Manipulating light to open up the future! Technology for measuring the unknown underwater world


Nanolux co. ltd.
Deliver Color Images in Darkness! Unique Technology Enabling Color Image only by Infrared Illumination


SteraVision Co.,Ltd.
Realizing an Eye that can See like a Human!
– Development of Optical Steering Device and New Standard Lidar -


ProteoBridge Corporation
Reproduce human on the palm !
-Development of the world's first human protein array-


Site Sensing Inc.
Expanding a New Business with Excellent Measurement Technologies for Tracking, 3D Modeling , and Face Recognition


Hmcomm Inc.
Controlling Voice to Open Up Next Generation Business!
- Commercializing AI and Voice Recognition Technology -
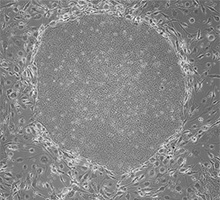

TOKIWA-Bio Inc.
Automatic Generation of iPS Cells!
- Contributing to Gene Therapy Worldwide -


HSP Technologies Inc.
Producing New Materials by Mixing Materials that do can not Mix!


MIRAISENS,Inc.
A feeling as if things were really there!
~ Digitization of physicality and bodily sensations ~

PicoTherm Corporation
Supporting information society by technology to measure thermophysical properties


EDP Co.
Dedication to the ultimate material, diamond
