1. Chemical linkers for post-synthetic modifications of oligonucleotides
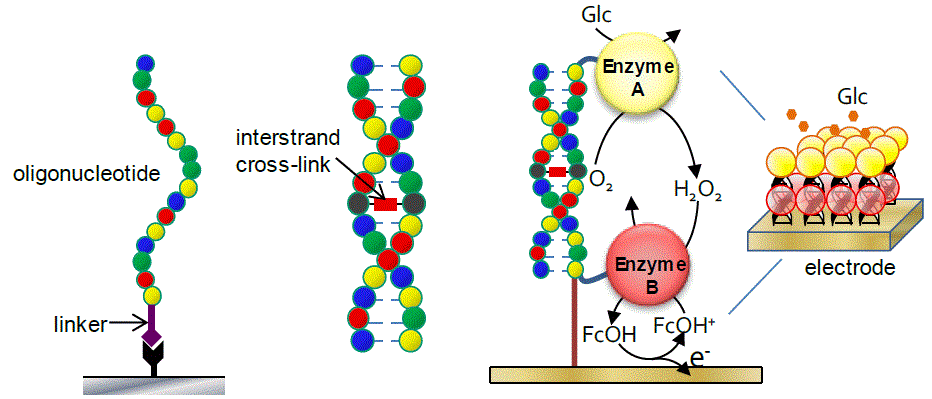
Chemical modifications of oligonucleotides are frequently for genetic analyses or oligonucleotide therapeutics. Chemical linkers are essential for covalent bonding between oligonucleotides and exogenous molecules. We have developed highly reactive amino-linkers for terminal modifications of oligonucleotides. These linkers enable high-throughput purification and high-yield modifications of functional oligonucleotides. We have also developed interstrand cross-linkers that can site-specifically conjugate a complementary duplex. Cross-linked duplexes form extraordinarily stable helices, enabling enzyme immobilization on electrode and construction of therapeutic agents.
Terminal modification and interstrand cross-link of oligonucleotids.
2. Electrochemical control of enzyme catalysis and evaluation of cellular reactios
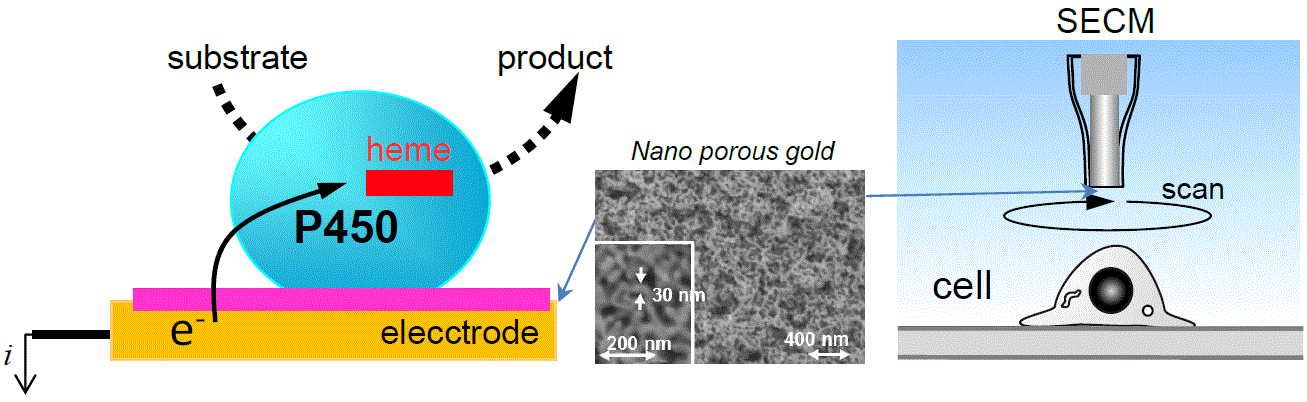
Cytochrome P450 (CYP) receives electrons to catalyze monooxygenation for a variety of substrates. We are developing the electron supplying method from an electrode to CYP immobilized on it, in order to monitor or utilize the CYP catalysis. We have proven that the nanostructured electrode surface (e.g. nanoporous gold) promotes the efficient electron transfer of the enzyme. We are seeking more effective electrodes and CYP-immobilization techniques.
We also focus on single-cell analyses, and have developed scanning electrochemical microscopy (SECM) enabling long-term monitoring of cellular topography or activities. We are further developing a novel SECM system to carry out single-cell analysis more conveniently.
Electrochemically-driven CYP reaction and cell kinetics observation by SECM.
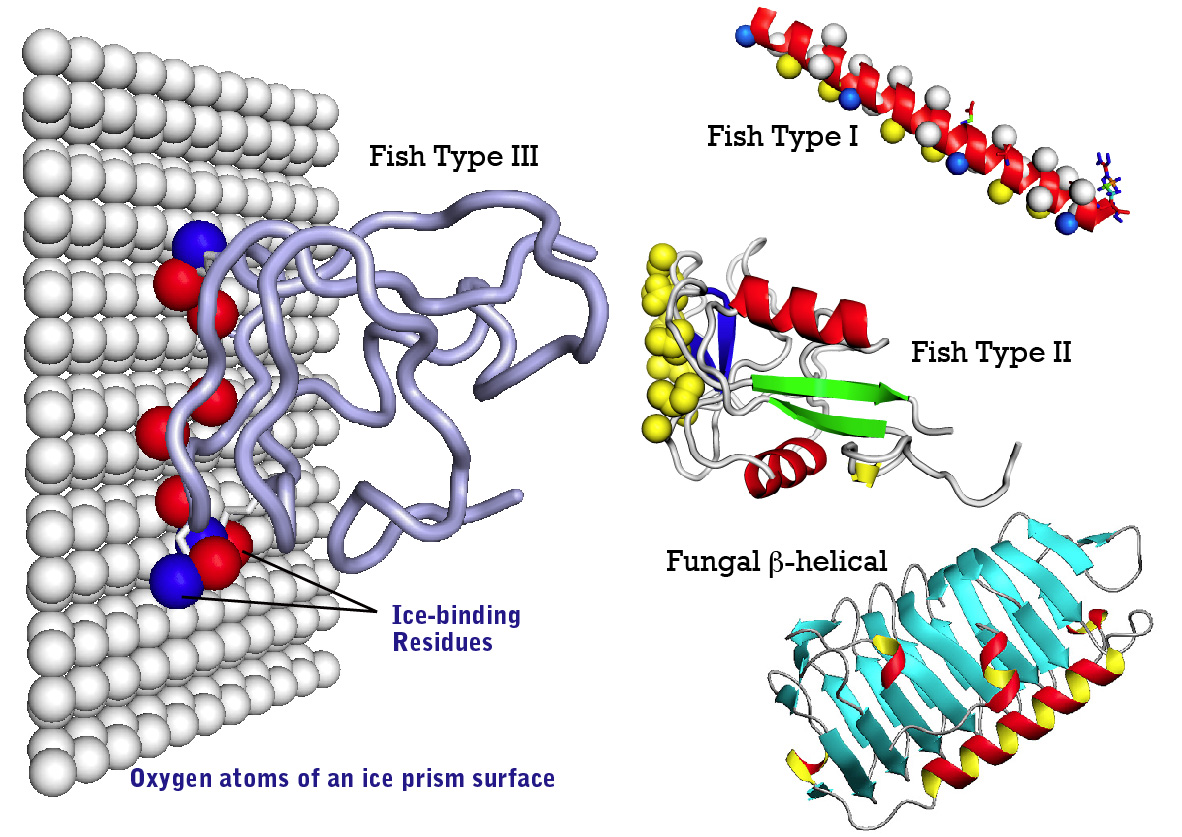
3. Analysis of structural base and ice-binding mechanism of AFP
We are trying to analyze structure-function relationship of natural - and recombinant AFPs and its activity-improved mutant as well. We are especially employing advanced techniques of molecular biology, biochemistry, ice physics, and structural biology (NMR and X-ray), all of which provides crucial information about structural base of this protein.
4. Development of AFP-utilizing technology
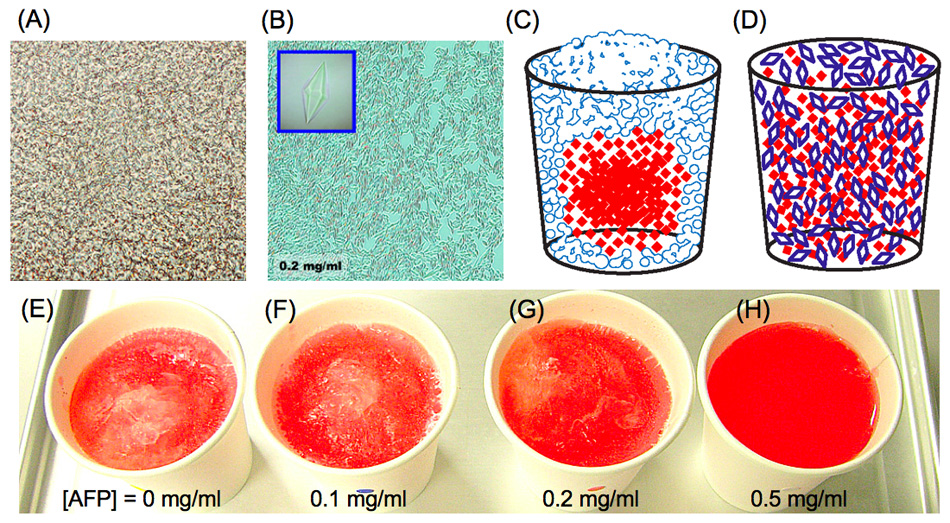
The following shows photomicroscope snapshots of (A) an ordinary ice block and (B) a frozen AFP-solution. The (A) looks like a 'concrete fluid' with numerous bubbles. This concrete expands with time and phisically destroys the inside of the frozen material. In contrast, the ice block containing 0.2-0.5mg/ml of AFP becomes an assembly of numerous tiny ice bipyramids (B). The ice crystals in (B) are hardly sticked together, and that they have less power to destroy an inside structure of the frozen material. Such an AFP-induced effect was verified by simple experiments using a colored water. When it freezes a water containing red-colored ink, the ink particles are excluded from the concrete ice (solid phase) to be concentrated into a center portion as sketched like (C). This is generally called "freeze-concentration phenomenon". In contrast, an entirely red-colored ice is created when the solution contains AFP, as sketched like (D). The photos (E) - (H) show the actual photos of the frozen colored ice prepared in a home freezer (-18 deg.) by overnight freezing. Each contains 0, 0.1, 0.2, and 0.5mg/ml of AFP, respectively. The power of such "freeze-concentration-inhibition" might be correlated with the TH value of AFP.