Mesoscopic Materials Group
Group Outline
We develop advanced functional materials toward promoting flexible and printed electronics. Main subjects are
to develop solution-processible electronic materials, innovative printing processes, and advanced measurement
techniques for improving performance of printed devices. We also try to develop new materials function such as
biocompatibility, antivirus, and antibacterial functions, as well as to adapt materials informatics and
process informatics to solution-processible electronic materials.
Key Themes of Research
- Developing new functions and solution-processibility for organic semiconductors, organic ferroelectrics,
two-dimensional layered materials, metal nanoparticles, and photo-functional materials.
- Developing innovative printing processes to realize high performance, high durability, and homogeneous characteristics for printed electronic devices.
- Developing advanced measurement techniques for quality improvement of printed devices, as well as modifying these techniques toward advanced biosensing.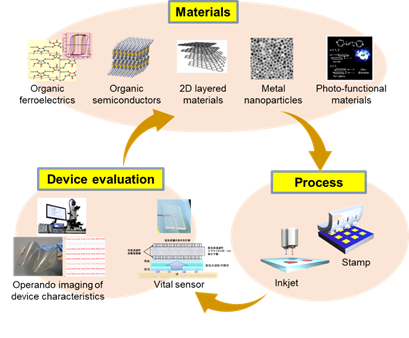
- Developing innovative printing processes to realize high performance, high durability, and homogeneous characteristics for printed electronic devices.
- Developing advanced measurement techniques for quality improvement of printed devices, as well as modifying these techniques toward advanced biosensing.

Major Achievements
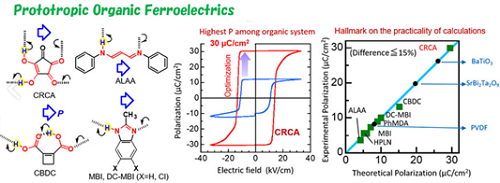
1.Organic ferroelectrics
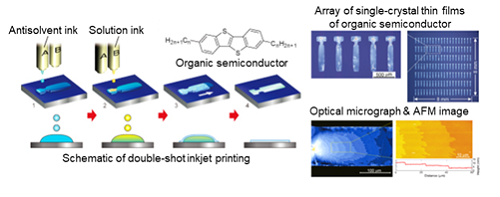
2.Printing single-crystal thin films of organic semiconductor
3. Printing ultrafine conductive pattern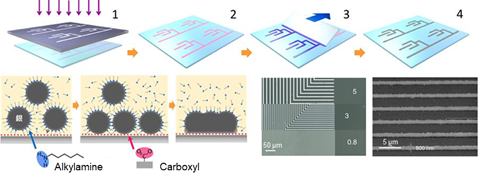
4.Visualization of device operation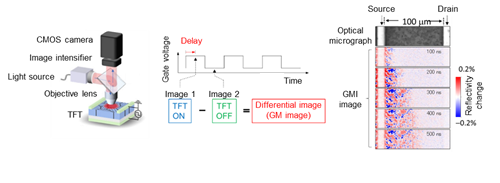

Organic ferroelectric material with world highest polarization (30 μC / cm2)

Double-shot inkjet printing of single-crystal OTFTs with the mobility higher than amorphous silicon.
3. Printing ultrafine conductive pattern

SuPR-NaP method enabling spontaneous formation of ultrafine (0.8 μm) conductive silver pattern
4.Visualization of device operation

Time-resolved gate modulation imaging technique unvailing microscopic charge transport dynamics in thin-film transistor.
Our Technologies and Equipment
Organic synthesis, Thin film deposition techniques (vacuum deposition, inkjet printing, blade coating, spin
coating), Calorimetry, X-ray diffraction analysis, Electric measurement (transistor characteristics,
ferroelectric characteristics, impedance spectroscopy), Electrochemical measurement, Microspectrophotometry
(UV-vis, infrared, Raman), Fluorescence spectroscopy, Modulation imaging, Scanning probe microscopy

