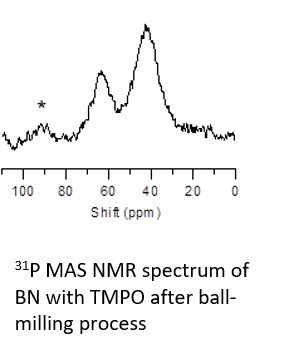Solid-State Nuclear Magnetic Resonance Spectrometer(SSNMR) SSNMR is used to measure the local structure and motion of atoms and molecules in solid materials by using atomic nuclear spin as a probe.
Equipment
Equipment 1
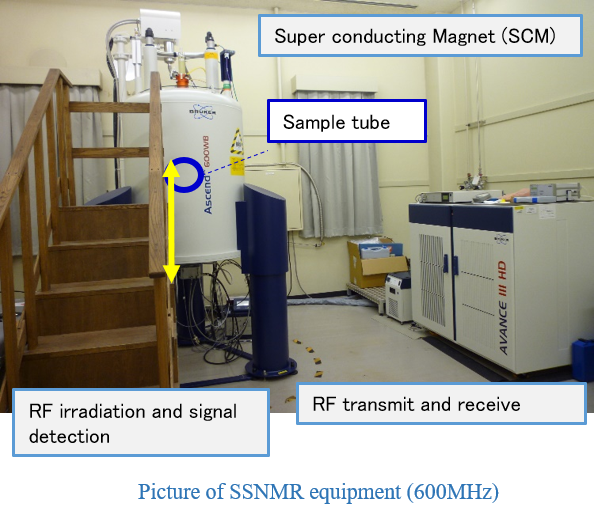
Solid-state NMR(600MHZ)for 1H-207Pd,Wide bore SCM type,1D and 2D experiments.Equipment 2
Solid-state NMR(200MHZ)for 1H-207Pd,Wide bore SCM type,1D and 2D experiments.Equipment 3
Solid-state NMR(20MHZ)for 1H only, Permanent magnet type, relaxation time measurement.Applications
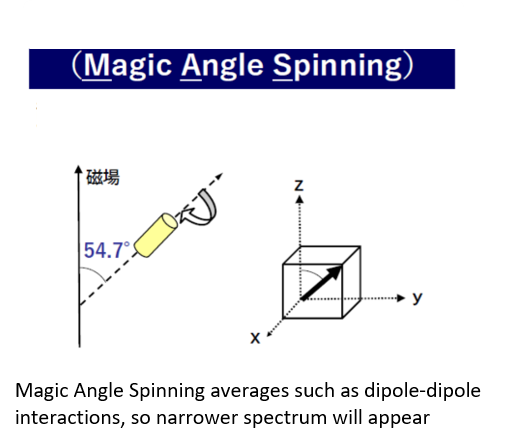
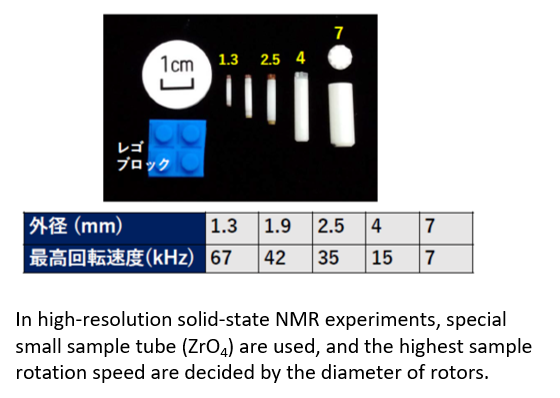
・Detects solid-state nuclear magnetic resonance with high resolution by circulating a solid sample at high speed.(0-60KHz)
・Used for in-situ measurement of materials (polymer, rubber, protein) whose structures can change when solved into a solvent.
・Analyzes the molecular bonding state of gaseous, solid, and semi-solid samples.Principle and Features
・Materials that possess nuclear spins in a strong magnetic field absorb radio-frequency (RF) waves. By using this phenomenon, it is possible to observe the spectrum in the RF region and analyze the averaged energy (chemical shifts) around nuclear spins.
・The solid-state NMR method can analyze the nanoscale structure of solid-state samples. It observes the dynamics of atoms, ions and molecules in a non-invasive, non-contact manner in the solid state.
Features of SSNMR
・Direct detection of the existence of the NMR active nucleus
・Non-invasive method for all materials.
・In principle, NMR is not a sensitive method, but high sensitivity is achieved by applying a strong magnetic field.
・It is difficult to conduct NMR experiments for magnetic samples.・Structure of light emitting materials (13C)
Examples
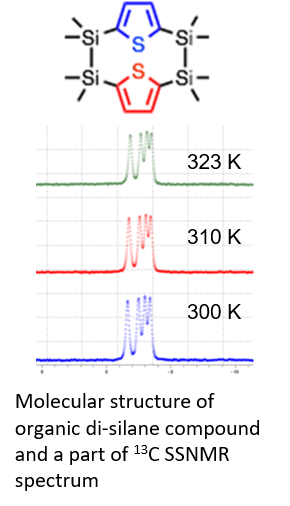
・Organic di-silane molecules are synthesized, and show a light emitting property and take various molecular conformations in the solution state. We have investigated molecular conformations in the solid state using solid-state NMR measurements. 13C CP/MAS NMR was performed at 150.97 MHz using a 4 mm sample tube and 8 kHz rotation speed. The figure shows the molecular structure of an organic di-silane compound and the temperature dependence and part of the 13C SSNMR spectrum. This shows that no conformation change occurs in solid-state samples.
・Acid-base property of BN catalyst (31P)
・Hexagonal boron nitride (BN) exhibits nitro-aldol reaction and Knoevenagel condensation reaction activity after the ball-milling process. Absorbing basic probe molecules, trimethylphosphineoxide (TMPO), 31P MAS NMR was performed at 161.98 MHz using a 4 mm sample tube and 8 kHz rotation speed. The figure shows the 31P MAS NMR spectrum: 63 ppm and 43 ppm signals were observed. The 43 ppm signal is assigned to physiosorbed TMPO and the 63 ppm signal is assigned to adsorption on acidic sites, based on chemical shift values.
・Organic-inorganic hybrid materials (13C)
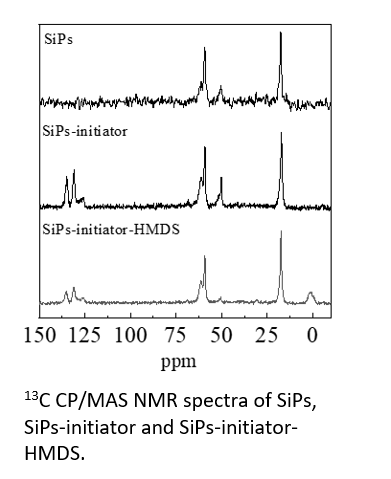
・The development of grafted silica particles of electrically conductive polymers was investigated. The figure shows the solid-state 13C CP/MAS NMR spectrum of silica particles in preparing grafted silica particle components, SiPs, SiPs-initiator and SiPs-initiator-HMDS. Capping of -OH was achieved by replacing -HMDS residue.
Other examples
・Structural analysis of metal hydrate, hydrogen sites and the diffusion process in hydrogen storage materials
・Interfacial structure and change of structure in the reversible melt-solidification reaction in inorganic and organic hybrid materials
・Mechanisms of conduction of batteries, ionic diffusion, and ion conducting materials
・Quantification of solid fat included in meats