
Development of the technologies for in-situ remediation of contaminated sites with low cost, low environmental impact remains a challenge for both scientists and engineers in the fields of geo-environment. R & D related to remediation technology has been performed at the Geo-Environmental Risk Research Group, AIST. A brief introduction of selected technologies is given below.
Purification technology using adsorbents for arsenic and other heavy metal contamination
In some parts of Asia and Africa, arsenic (As) contaminated groundwater is consumed directly from wells as drinking water, which can cause health problems. In addition, the large amounts of As-containing excavation waste generated during tunnel construction and other such work are also a major problem.Our group is conducting research aimed at developing inexpensive and effective adsorbents and their application technologies that can be used in economically disadvantaged developing countries or in large-scale pollution control construction projects. We are focusing on adsorbents based on magnesium (Mg)- and calcium (Ca)-based compounds as environmentally friendly adsorbents that do not have a negative impact on the human body or ecosystem, and are conducting detailed evaluations of As removal performance through various tests. Furthermore, there are many cases of mixed contamination caused by elution of fluorine (F) along with As from natural sedimentary layers and rocks. Our group is currently conducting research into the simultaneous removal and countermeasures of such mixed contamination using adsorbents.
As an example, Figure 1 shows the As removal rate RAS and F removal rate RF obtained in a simultaneous As-F removal test using a Mg-based adsorbent. Both RAS and RF increase with an increase in the adsorbent addition concentration WAd0/V, but it can be seen that the behavior of both differs depending not only on the type of adsorbent but also on the valence of As (trivalent and pentavalent). It has also been confirmed that both RAS and RF are affected by differences in the initial As concentration (CAS0), the initial F concentration (CF0) and initial pH (pH0).
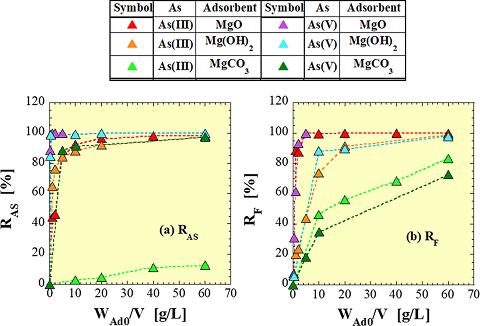
Fig. 1
(a) As removal ratio, (b) F removal ratio
Experimental conditions:
CAS0 = 1mg/L, CF0 = 60mg/L, pH0 = 7
On the other hand, the adsorbents used to purify As contamination are themselves contaminants that contain large amounts of As. Therefore, if spent adsorbents are not properly treated and disposed of, they may cause secondary contamination. In particular, in developing countries, spent adsorbents may be disposed of in the environment without proper treatment. Therefore, our group is conducting experiments simulating cases in which spent adsorbents are directly disposed of in soil, exposed to acid rain, or come into contact with groundwater or soil pore water of various pH levels, and is conducting a comprehensive environmental stability evaluation of spent adsorbents by investigating the elution behavior of As and parent material components from the spent adsorbents.
As an example, we prepared spent adsorbents by adsorbing As onto Mg- and Ca-based adsorbents, and compared the As elution rate (EAS) under various test conditions in Figure 2. We can see that the EAS varies greatly depending on the combination of the adsorbent type, As valence, initial pH of the solution, and soil type.
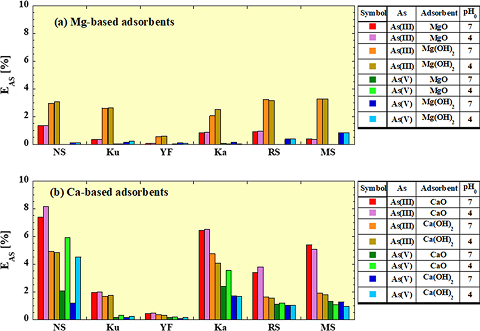
Fig. 2
Comparison of As leaching ratio for each soil: (a) spent Mg-based adsorbents, (b) spent Ca-based adsorbents
NS: No-soil, Ku: Kuroboku soil, YF: Yellow-brown forest soil, Ka: Kanuma soil, RS: River sand, MS: Mountain sand
Papers related to Figures 1 and 2
- 1) Sugita, H., Morimoto, K., Saito, T., Hara, J.(2024)Simultaneous removal of arsenate and fluoride using magnesium-based adsorbents. Sustainability, 16(5), 1774. https://doi.org/10.3390/su16051774
- 2) Sugita, H., Morimoto, K., Saito, T., Hara, J.(2024)Removal performance and adsorption behavior on Mg-based adsorbents in As(III) and F simultaneous removal as in comparison with As(V). Geochemistry: Exploration, Environment, Analysis, 24(4), geochem2024-022, 2024. https://doi.org/10.1144/geochem2024-022
- 3) Sugita, H,; Oguma, T., Zhang, M., Hara, J., Takahashi, S. (2016) Environmental stability of spent magnesium-based and calcium-based arsenic adsorbents-Effects of soils. Journal of Japan Society of Civil Engineers, Ser. G (Environmental Research), 72, III_437–III_448. https://doi.org/10.2208/jscejer.72.III_437
- 4) Sugita, H., Morimoto, K., Saito, T., Hara, J.(2024)Effects of soils on environmental stability of spent Mg-based and Ca-based adsorbents containing arsenite. Sustainability, 16(10), 4008. https://doi.org/10.3390/su16104008
Bioremediation
approaches for cleaning up the sites polluted by organic contaminants, such as chlorinated ethenes. Although bioremediation, in its widest sense, is not new, and many researches have been performed on bioremediation of different kinds of pollutants, an effective design and implication of in situ bioremediation still remains a challenging problem because of the complexity.
Many factors may affect the applicability and efficiency of bioremediation of chlorinated ethenes in situ, which include the type and concentration of contaminants, biological, geological and hydro-geological conditions of the site, physical and chemical characteristics of groundwater and soils to be treated, as well as the constraints in engineering.
We are conducting research and development that combines soil and groundwater survey, geochemical analysis, microbial incubation, molecular biological experiment, and bioinformatics. We have been able to obtain design conditions such as the optimal conditions and application limits for bioremediation using anaerobic microorganisms to dechlorinate chloroethenes such as tetrachloroethene (PCE) and trichloroethene (TCE). For example, we have demonstrated that chloroethenes can be dechlorinate into harmless compounds in a short period of time by optimizing parameters such as ferrous iron and methanogenic archaea (Figure 1, AIST HP). We are also studying on the degradation of benzene and dichloromethane by aerobic microorganisms.
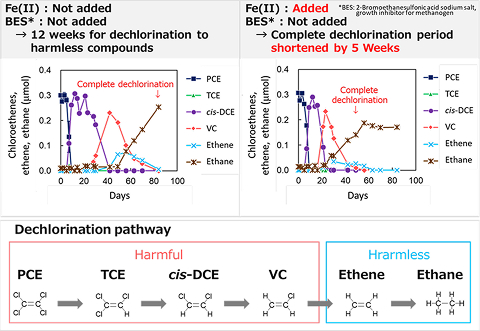
Fig. 1
Results of laboratory experiments on anaerobic dechlorination of chloroethenes by microorganisms
Papers
- Miho Yoshikawa, Ming Zhang, Yoshishige Kawabe, Taiki Katayama (2021) Effects of ferrous iron supplementation on reductive dechlorination of tetrachloroethene and on methanogenic microbial community. FEMS microbiology ecology, 97(5), 12. https://doi.org/10.1093/femsec/fiab069
- Miho Yoshikawa, Ming Zhang, Futoshi Kurisu, Koki Toyota (2017) Bacterial Degraders of Coexisting Dichloromethane, Benzene and Toluene, Identified by Stable-Isotope Probing. Water, Air, & Soil Pollution, 228(11), Article: 418. https://link.springer.com/article/10.1007/s11270-017-3604-1
Remediation of Organic Compounds Using Minerals
Compared to chemical materials, natural minerals are generally cheaper and environmentally-friendly. Taking these advantages over chemical materials, studies on remediation of organic compounds using minerals have been performed.
Iron sulfide ores were found to exhibit oxidative degradation properties against toxic organic components, trichloroethylene, chlorobenzene, and pesticide residues that accumulate in the soil environment. This is because the chemical degradation is attributed to hydroxyl radicals generated on the surface of the sulfide, which shows oxidative power to open benzene rings as well as linear chains (Fig.1).
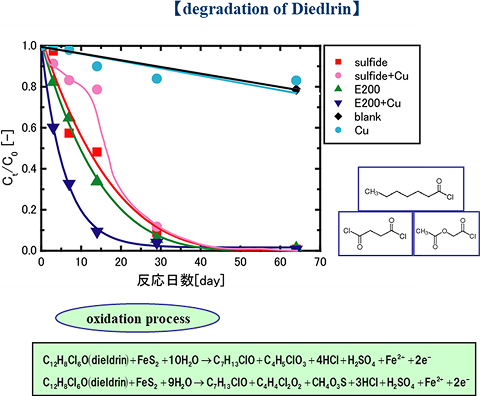
Fig. 1
Comparison between decomposition speed of Dieldrin by natural minerals and iron powder
Iron sulfide ores are widely distributed in coastal areas inhabited by mangroves in East and Southeast Asia, where these pollutants are of particular concern, and may be preventing the marine discharge of toxic substances generated on land. In this way, elemental cycling of iron and sulfur in coastal areas is expected to be effective through environmental conservation and natural attenuation in coastal areas(Fig.2).
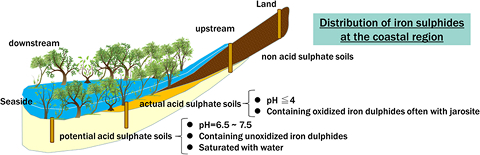
Fig. 2
Potential acid sulphate soils formed in coastal regions, especially in the tropical to subtropical zone of East Asia and Southeast Asia
Papers
- Hara J. (2012) Chemical degradation of chlorinated organic pollutants for In Situ Remediation and Evaluation of Natural Attenuation, Organic Pollutants, InTech Press, pp.345-364.
- Hara J. (2011) The effect of oxygen on chemical dechlorination of dieldrin using iron sulphides,Chemosphere,82,pp.1308-1313.
- Hara J. (2017) Oxidative degradation of benzene rings using iron sulfide activated by hydrogen peroxide/ozone. Chemosphere, 189, 382-389.
Acid wastewater treatment technology using recycled materials
In Japan, there are rivers that have acidic water quality due to mine wastewater from abandoned mines and the inflow of acidic hot spring water. Some abandoned mines are continuously producing acidic mine wastewater, and countermeasures are necessary. In particular, mine wastewater often contains heavy metals such as arsenic, iron, cadmium, and lead, so there are concerns that discharging mine wastewater or acidic hot spring water into rivers will pollute the river and have a negative impact on people, ecosystems, and the environment.
Currently, neutralization work is carried out using natural resources such as calcium carbonate to prevent impacts on the ecosystem, but the high cost and environmental impact are problems. Therefore, we are conducting research with the aim of using recycled materials such as oyster shells and recycled concrete as alternative neutralizing agents.

Fig. 1
Rivers into which acid mine wastewater flows
Towards practical applications, systematic studies on different kinds of adsorbents are now in progress. Many other technologies for practical remediation of the sites contaminated with heavy metals, and VOCs are available at our research group.

