Methane Hydrate Production Techology Group
Japanese / English
RESEARCH: Realizing a Low-carbon Energy from Hokkaido

Gas clathrate hydrates (gas hydrates) are clathrate compounds that crystallize by enclosing guest molecules in water structures. Because gas hydrates are formed under high-pressure, low-temperature conditions, natural gas hydrates are found in oceanic and permafrost sediments. Guest molecules in natural gas hydrates are almost exclusively methane; Methane obtained from natural gas hydrates is considered to be a potential new unconventional energy resource.
1. Natural Hydrate Bearing (NGH) Sediments
The permeability, stiffness, and thermal conductivity of NGH sediments are important characteristics determining the gas production from a gas hydrate reservoir. AIST joins the Research Consortium for Methane Hydrate Resources in Japan (MH21-S Research Consortium), planned by the Ministry of Economy, Trade, and Industry (METI), Japan, and develops pressure-core nondestructive analysis tools (PNATs) for analyzing hydrostatic pressurized natural GH sediments. The PNATs essentially comprised a manipulator for core-cutting and transferring (PNATs-MAN) and the IPTC (PNATs-AIST-IPTC) systems. The PNATs-MAN and PNATs-AIST-IPTC were installed in a cold room kept at a controlled temperature of 278−283 K. The basic PNATs components were developed in collaboration with Georgia Tech and USGS.

Fig.1 Pressure-core nondestructive analysis tools (PNATs)
AIST studies NGH sediments recovered from the eastern Nankai area and other are in Japan by using PNATs. We collaborated other country partner to measure sedimental propteries of NGH oceanic sediment from area except for Japan (eg. Indian ocean)
Related work:e.g. Yoneda et al., Mar. Pet. Geol., 2015, 66,451-459.
2. Using the nature of clathrate hydrates
Thermal and cold energy storage
Latent heat storage systems using semiclathrate hydrates as phase-change materials (PCMs) are efficient systems for thermal and cold energy storage. In order to fully understand their thermal stabilities and to gain insight into the characterization of semiclathrate hydrates, we investigated relationship between thermal properties and crystal structures using phase equilibrium measurements, differential scanning calorimetry (DSC), and powder X-ray diffraction (PXRD).
Related work:e.g. Oshima et al., J. Chem. Thermodynamics, 2018, 123, 32-37.
Gas storage and transportation
Gas hydrates can store high volumes of gas: 170 times the volume of a guest gas molecule is stored in a unit of gas hydrate. Thus, gas hydrates are of interest as a gas storage medium. For establishment of natural gas storage using gas hydrate technology, we study self-presevation phenomenon during gas hydrate dissociation.
Related work:e.g. Kida et al., Jpn. J. Appl. Phys., 2017, 56, artNo. 095601.
Guest-host interaction and phase transition
We also study guest-host interaction of sH hydrates using Raman spectroscopy, and investivate newly large-molecule guest substances and its crystal properties.
Related work:e.g. Jin et al., J. Phys. Chem. C, 2015, 119, 9069–9075.
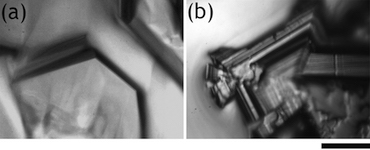
Fig.3 Crystal phase transition of semiclathrate hydrate (scale bar: 100 μm)
(a)P4/mmm →(b)Pmma
(Reprinted with permission from J. Phys. Chem. C, 2013, 117 (14), pp 6924–6928. Copyright 2013 American Chemical Society.)
PUBLICATIONS
2019
Y. Jin, M. Kida, J. Yoneda, Y. Konno, N. Tenma, and J. Nagao, "Natural Gas Hydrates Recovered from the Umitaka Spur in the Joetsu Basin, Japan: Coexistence of Two Structure-I Hydrates with Distinctly Different Textures and Gas Compositions within a Massive Structure", ACS Earth Space Chem., 2020, 4, 77-85.
DOI: 10.1021/acsearthspacechem.9b00249 (OPEN ACCESS)
M. Oshima, Y. Jin, M. Kida and J. Nagao, "Thermodynamic and crystallographic properties Depending on hydration numbers in tetra-n-butylammonium chloride semiclathrate hydrates", J. Chem. Thermodynamics, 2020, 142, 106004.
DOI: 10.1016/j.jct.2019.106004
J. Yoneda, M. Kida, Y. Konno, Y. Jin, S. Morita, and N. Tenma, "In situ mechanical properties of shallow gas hydrate deposits in the deep seabed", Geophys. Res. Lett., 2019, 46, 14459-14468.
DOI: 10.1029/2019GL084668 (OPEN ACCESS)
Y. Jin, M. Kida and J. Nagao, "Structure H Clathrate Hydrates in Methane–Halogenic Large Molecule Substance–Water Systems", J. Phys. Chem. C, 2019, 123, 17170-17175.
DOI: 10.1021/acs.jpcc.9b04691
Y. Jin, M. Kida and J. Nagao, "Crystal Phase Conditions on Semi-clathrate Hydrates in Nitrogen–Tetra-n-butylammonium Bromide–Water Systems below 1 MPa", J. Chem. Eng. Data, 2019, 64, 2843-2848.
DOI: 10.1021/acs.jced.9b00210
M. Kida, Y. Jin, and J. Nagao, "Changes in the 13C NMR Spectra of Tetra-n-butylammonium Chloride by Clathrate Hydration", Chem. Phys., 2019, 522, 233-237.
DOI: 10.1016/j.chemphys.2019.03.010
M. Oshima, K. Suzuki, J. Yoneda, A. Kato, M. Kida, Y. Konno, M. Muraoka, Y. Jin, J. Nagao, and N. Tenma, "Lithological Properties of Natural Gas Hydrate–bearing Sediments in Pressure-cores Recovered from the Krishna–Godavari Basin", Mar. Pet. Geol., 2019, 108, 439-470.
DOI: 10.1016/j.marpetgeo.2019.01.015
2018
A. Kato, Y. Konno, J. Yoneda, M. Kida, M. Oshima, Y. Jin, J. Nagao, and N. Tenma, "Evaluation of Failure Modes and Undrained Shear Strength by Cone Penetrometer for Natural Gas Hydrate-bearing Pressure-core Sediment Samples Recovered from the Krishna-Godavari Basin, Offshore India", Mar. Pet. Geol., 2019, 108, 502-511.
DOI: 10.1016/j.marpetgeo.2018.11.015
M. Kida, Y. Jin, J. Yoneda, M. Oshima, A. Kato, Y. Konno, J. Nagao, and N. Tenma, "Crystallographic and Geochemical Properties of Natural Gas Hydrates Accumulated in the National Gas Hydrate Program Expedition 02 Drilling Sites in the Krishna-Godavari Basin off India", Mar. Pet. Geol., 2019, 108, 471-481.
DOI: 10.1016/j.marpetgeo.2018.10.012
Y. Konno, A. Kato, J. Yoneda, M. Oshima, M. Kida, Y. Jin, J. Nagao, and N. Tenma, "Numerical Analysis of Gas Production Potential from a Gas-hydrate Reservoir at Site NGHP-02-16, the Krishna–Godavari Basin, Offshore India–Feasibility of Depressurization Method for Ultra-deepwater Environment", Mar. Pet. Geol., 2019, 108, 731-740.
DOI: 10.1016/j.marpetgeo.2018.08.001
J. Yoneda, M. Oshima, M. Kida, A. Kato, Y. Konno, Y. Jin, J. Jang, W. Waite, P. Kumar, and N. Tenma, "Pressure Core Based Onshore Laboratory Analysis on Mechanical Properties of Hydrate-bearing Sediments Recovered during India's National Gas Hydrate Program Expedition (NGHP) 02", Mar. Pet. Geol.,2019, 108, 482-501.
DOI: 10.1016/j.marpetgeo.2018.09.005
J. Yoneda, M. Oshima, M. Kida, A. Kato, Y. Konno, Y. Jin, and N. Tenma, "Consolidation and Hardening Behavior of Hydrate-bearing Pressure-core Sediments Recovered from the Krishna–Godavari Basin, Offshore India", Mar. Pet. Geol.,2019, 108, 512-523.
DOI: 10.1016/j.marpetgeo.2018.09.021
J. Yoneda, M. Oshima, M. Kida, A. Kato, Y. Konno, Y. Jin, J. Jang, W. Waite, P. Kumar, and N. Tenma, "Permeability Variation and Anisotropy of Gas Hydrate-bearing Pressure-core Sediments Recovered From the Krishna–Godavari Basin, Offshore India", Mar. Pet. Geol.,2019, 108, 524-536.
DOI: 10.1016/j.marpetgeo.2018.07.006
M. Oshima, M. Kida, and J. Nagao, "Hydration Numbers and Thermal Properties of Tetra-n-butylammonium Bromide Semi-clathrate Hydrates Determined by Ion Chromatography and Differential Scanning Calorimetry", J. Chem. Thermodynamics, 2018, 123, 32-37.
DOI: 10.1016/j.jct.2018.03.018
J. Yoneda, A. Takiguchi, T. Ishibashi, A. Yasui, J. Mori, M. Kakumoto, K. Aoki, and N. Tenma, "Mechanical Reaction of Reservoir and Well Completion of the First Offshore Methane Hydrate Production Test at the Eastern Nankai Trough: A Coupled Thermo-Hydro-Mechanical Analysis", SPE J., 2019, 24, SPE-191145-PA.
DOI: 10.2118/191145-PA
2017
M. Kida, Y. Jin, M. Watanabe, T. Murayama, and J. Nagao, "Improvement of Gas Hydrate Preservation by Increasing Compression Pressure to Simple Hydrates of Methane, Ethane, and Propane", Jpn. J. Appl. Phys., 2017, 56, 095601.
DOI: 10.7567/JJAP.56.095601
Y. Konno, T. Fujii, A. Sato, K. Akamine, M. Naiki, Y. Masuda, K. Yamamoto, and J. Nagao, "Key Findings of the World's First Offshore Methane Hydrate Production Test off the Coast of Japan: Toward Future Commercial Production", Energy & Fuels, 2017, 31,2607–2616.
DOI: 10.1021/acs.energyfuels.6b03143
Y. Jin, M. Kida, Y. Konno, and J. Nagao, "Clathrate Hydrate Equilibrium in Methane–Water Systems with the Addition of Monosaccharide and Sugar Alcohol", J. Chem. Eng. Data, 2017, 62 440-444.
DOI: 10.1021/acs.jced.6b00756
MEMBERS
| AIST lab member |
| Yusuke JIN |
Group leader |
Spectroscopy, Solution Chemistry |



|
| Jun YONEDA |
Resercher |
Geotechnical engineering, Numerical simulation |


 |
| Motoi OSHIMA |
Resercher |
Physical Chemistry, Thermodynamics |


 |
| Collaborator |
| Masato KIDA |
Kitami Institute of Technology
Assistant Professor |
Physical Chemistry |



|
| Yoshihiro KONNO |
The University of Tokyo
Associate professor |
Reservoir engineering
|



|



