Geo-environmental Risk Research Group
JapaneseRemediation Technology
Development of the technologies for in-situ remediation of contaminated sites with low cost, low environmental impact remains a challenge for both scientists and engineers in the fields of geo-environment. R & D related to remediation technology has been performed at the Geo-Environmental Risk Research Group, AIST. A brief introduction of selected technologies is given below.
Electro-kinetic Remediation Technology
The electrokinetic remediation variably called electroreclamation, electrokinetic soil processing and electrochemical decontamination, involves the installation of electrodes into the contaminated soil and application of low-intensity direct current through the soil to stimulate electrochemical and electrokenitic processes within the soil. The processes result in the generation of an acid front at the anode that eventually moves from the anode to the cathode, enhancing desorption and/or dissolution of metal contaminants from the soil matrix, and mobilizing the contaminants toward the electrodes for removal and/or treatment.
Although many bench-scale laboratory experiments have demonstrated that the electrokinetic approach can be applied to remove different kinds of metals, such as chromium (Cr ), lead (Pb ), Zinc (Zn ), Nickel (Ni ) and copper (Cu ), from a variety of soils, such as clay, sand and silt, the effectiveness of this approach is a function of the contaminant type and concentration, the type, structure and chemistry of soil, the electric potential difference (voltage gradient) and/or intensity of current, and the solutions used to control the pH and/or to enhance desorption of metals from soil matrix. Besides, most bench-scale experiments were performed under idealized conditions that may not be necessarily representative to those in situ. Furthermore, chemical components, such as acids, used for controlling the pH and enhancing desorption of contaminants may cause problems of harmful environmental impact.
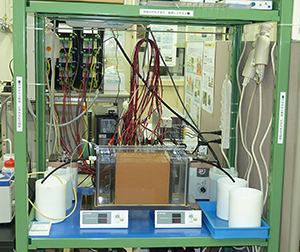
The key to the success of an electrokinetic remediation is the criteria for judging remediability and design conditions for implementation. To obtain the necessary knowledge capable of remediating contaminants from Japanese soils, an automated monitoring and controlling system for electrokinetic study has been developed (Fig. 1). Using this system, systematic experiments on different kinds of soils with different kinds of contaminant have been performed. Based on the knowledge obtained from the laboratory studies, an in situ pilot test using solar energy illustrated the applicability of our knowledge and the success of this technology (Fig. 2).
 |
 |
| a) Soil tank | b) Automated monitoring and controlling system |
Fig. 1 Laboratory system for electrokinetic remediation
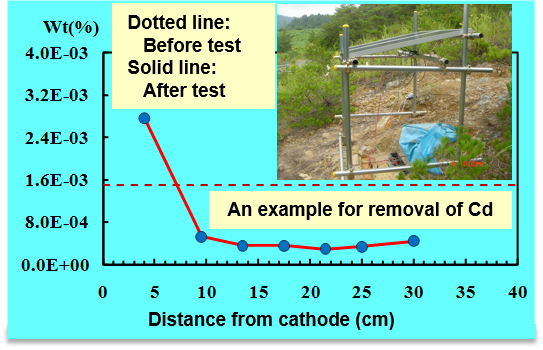
Fig. 2 A pilot test using solar energy for electrokinetic remediation in situ
Bioremediation
Bioremediation has been considered as one of environmentally friendly and cost effective approaches for cleaning up the sites polluted by organic contaminants, such as chlorinated ethenes. Although bioremediation, in its widest sense, is not new, and many researches have been performed on bioremediation of different kinds of pollutants, an effective design and implication of in situ bioremediation still remains a challenging problem because of the complexity.
Many factors may affect the applicability and efficiency of bioremediation of chlorinated ethenes in situ, which include the type and concentration of contaminants, biological, geological and hydro-geological conditions of the site, physical and chemical characteristics of groundwater and soils to be treated, as well as the constraints in engineering.
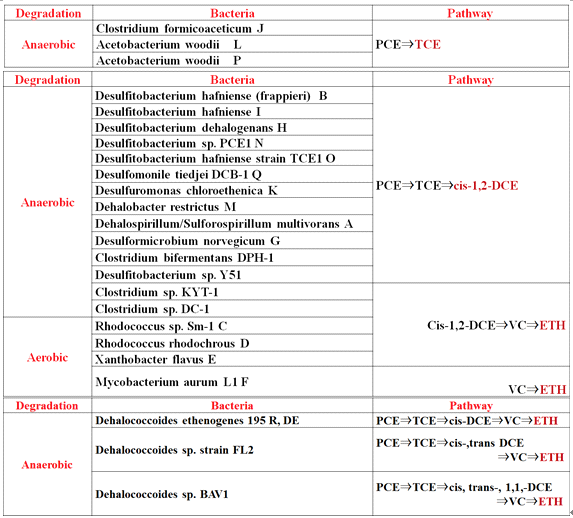
Focusing on the studies related to habitat conditions for the bacteria that are applicable for bioremediations, countermeasures to reduce the effects from limiting factors, conditions for enhancing bioremediations, we have obtained some systematic knowledge, especially those on bioremediation of the sites contaminated with PCE, TCE, cis-DCE and/or VC, and the sites contaminated with chloroethylenes and benzene.
Table 1 Degradation conditions, bacteria and
pathways for bioremediation of chlorinated ethenes
Remediation of Organic Compounds Using Minerals
Compared to chemical materials, natural minerals are generally cheaper and environmentally-friendly. Taking these advantages over chemical materials, studies on remediation of organic compounds using minerals have been performed.
Fig.1 shows the result of decomposition of TCE using pyrite and chalcopyrite, compared with those using iron powder. The results illustrated that the decomposition speed of TCE by pyrite or chalcopyrite is higher than by iron powder, meaning that higher efficiency can be obtained by using natural minerals.
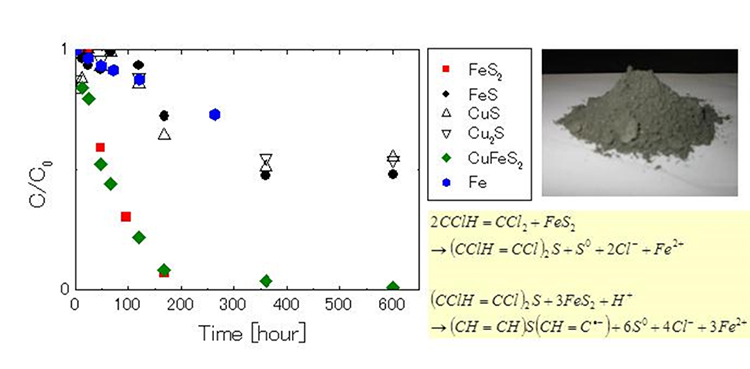
Fig. 1 Comparison between decomposition speed of TCE by natural minerals and iron powder
Besides the TCE, systematic experiments on decomposition of other kinds of organic components such as chlorobenzene and residual agricultural chemicals are now ongoing now.
Peer-reviewed papers:
Hara J. (2012) Organic Pollutants, InTech Press, pp.345-364.
Hara J. (2011) Chemosphere,82,pp.1308-1313.
Treatment of Drinking Water Polluted with Arsenic Using Adsorbents
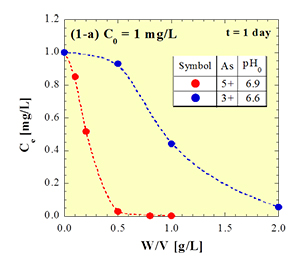
In some areas of Southeast Asia, South Asia and South America, groundwater contaminated by arsenic has being directly utilized as drinking water from wells, and may induce potential impact on human health. The purpose of this study is to develop low-cost adsorbents that can be practically used in developing countries to remove arsenic from arsenic contaminated water.
Fig. 1 illustrates the decrease in arsenic concentration in artificially polluted water accompanying with stepwise addition of very small amount of a newly developed adsorbent. Both the As3+ and As5+ can be removed by the adsorbent, and the efficiency is higher for As5+ compared with As3+.
 |
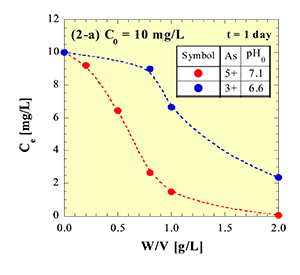 |
| a) Initial concentration 1 mg/L | b) Initial concentration 10 mg/L |
Fig. 1 An example of adsorption tests for removal of As
Towards practical applications, systematic studies on different kinds of adsorbents are now in progress.
Many other technologies for practical remediation of the sites contaminated with heavy metals, and VOCs are available at our research group.





