Sub-THz radiation nonthermally affects protein dynamics
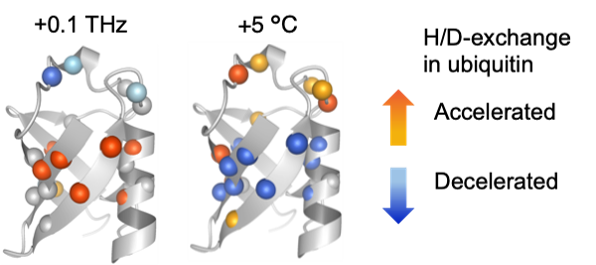
At physiological temperatures in aqueous solution, fluctuating dynamics of protein and coupled water molecules occur in the sub-terahertz (THz) frequency range. Combining the sub-THz irradiation with NMR-based measurements at the atomic level, Tokunaga et al. demonstrated that the applied sub-THz radiation energy induces structural dynamic changes of ubiquitin opposite to those induced by increased temperature (Figure 1). This result suggests that the heterogeneous water dynamics occurring at the protein-water interface include components that are nonthermally excited by the sub-terahertz radiation. This also raises the possibility that the sub-THz wave could be an effective tool for controlling protein functions in aqueous solutions.
Fig. 1. Effect of 0.1 THz irradiation (left) and temperature increase (right) on the amide proton exchange of ubiquitin.
Reasearch Background
Conformational heterogeneity driven by thermal fluctuations plays crucial roles for expressing protein functions. However, many fundamental issues remain unresolved, including how heterogeneous hydration dynamics at the protein-water interface contribute to the functions.
Recent spectroscopic studies have shown that a hydrated protein contains multiple components of diffusional and vibrational dynamics of water and protein, which may be coupled in sub-THz frequency range at physiological temperatures. Thus, one can hypothesize that intense sub-THz radiation may directly excite biologically relevant protein-water-coupled dynamics and may show one about the microscopic information of the dynamics. To date, little is known of whether the externally applied alternating electromagnetic field with the (sub)-THz frequency can resonantly interact with protein and hydration dynamics.
To address this issue, Imashimizu and his colleagues in AIST have started ‘Terabiology’, aiming to understand biomolecular functions based on biomacromolecule-water-coupled dynamics in the (sub)-THz frequency ranges, developing novel approaches that combine various types of THz wave sources and probing techniques for biomolecular dynamics and reactions.
Research Content
Tokunaga et al. demonstrated that klystron-based 0.1THz irradiation significantly influenced slow ubiquitin dynamics in a manner that cannot be explained by temperature increase (Figure 1). This finding was obtained at the atomic level in aqueous solution by developing their original approach combining the THz irradiation with NMR-based H/D exchange measurement (Figure 2). This result suggests that the direct excitation by the sub-THz radiation energy occurred in the heterogenous hydrogen-bond network around ubiquitin. This finding would also be a key to understand the role of protein-water-coupled dynamics for a wide range of protein functions.
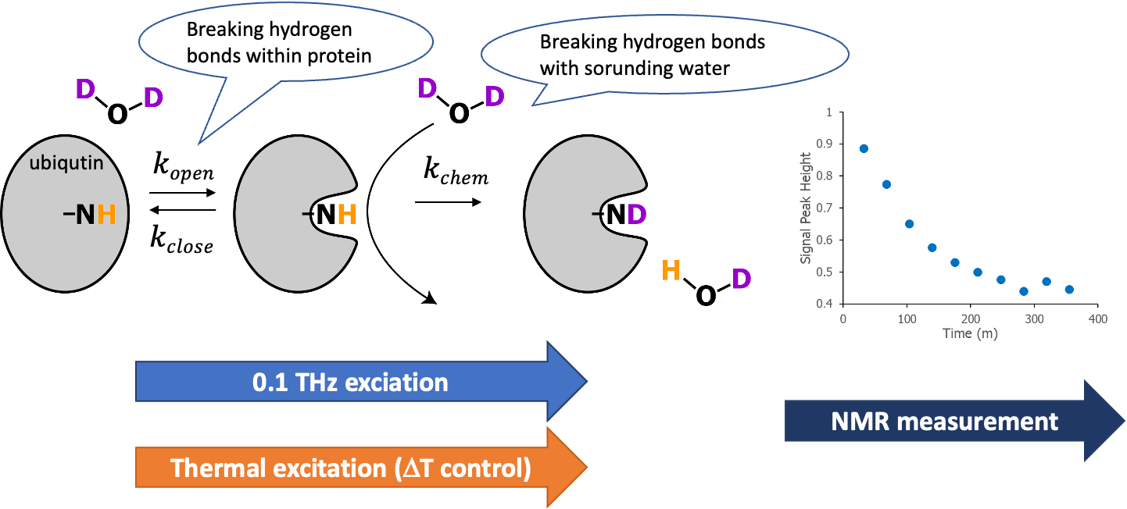
Fig. 2. THz-HDX method, combining a klystron-based 0.1-THz irradiation system with NMR-based hydrogen-deuterium exchange (HDX). Ubiquitin was used as a model protein. When the solvent is D2O, the main chain amide proton is replaced with deuteron upon opening, then H NMR signal disappears. Both the protein opening and chemical exchange processes require breaking of hydrogen bonds. Therefore, this method is highly sentive to the changes in hydrogen bond network induced by sub-THz exciation. A control experiment just by increasing temperature by heat conduction without 0.1-THz radiation was also performed.
Reference
- Title: Nonthermal excitation effects mediated by subterahertz radiation on hydrogen exchange in ubiquitin
- AAuthors: Y. Tokunaga, M. Tanaka, H. Iida, M. Kinoshita, Y. Tojima, K. Takeuchi and M. Imashimizu* (* corresponding author)
- Journal: Biophysical Journal, April, 21 (2021)
- DOI: 10.1016/j.bpj.2021.04.013