Cellular Presymptomatic Engineering Research Group
Cellular Presymptomatic Engineering Research Group Overview
We are exploring functional ingredients that improve aging and the associated sarcopenia, and elucidating their mechanisms of action. We are also searching for biomarkers that contribute to the early detection of mental disorders such as sleep disorders and depression. By integrating multiple cellular and animal models, we are promoting industry-academia-government collaboration to extend healthy life expectancy.
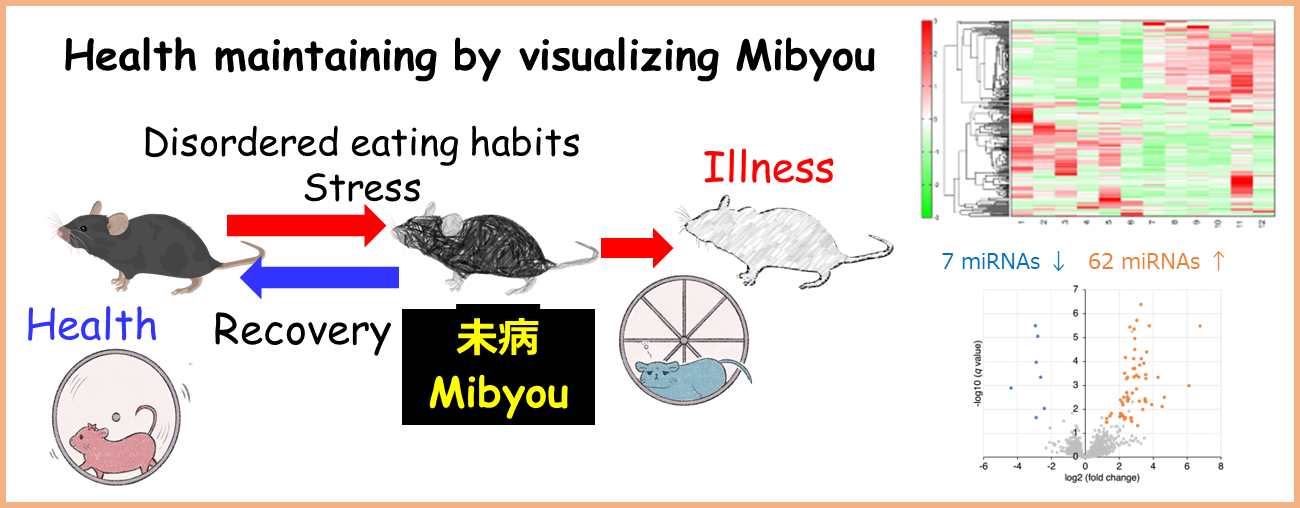
Research Project
Project 1:Development of evaluation techniques for the prevention of neurological dysfunctions associated with aging
Researcher: SHINKAI Yoichi
Aging is a risk factor for various neurological diseases such as ALS and Alzheimer's disease. In recent years, there has been a growing interest in the development of methods to control and visualize liquid-liquid phase separation for the fundamental treatment and early diagnosis of neurological diseases associated with this aging process. Using the short-lived nematode C. elegans, I am developing a high-throughput evaluation system for human cells. In addition, I am also exploring functional substances that contribute to disease prevention and preemptive measures by utilizing these evaluation systems.
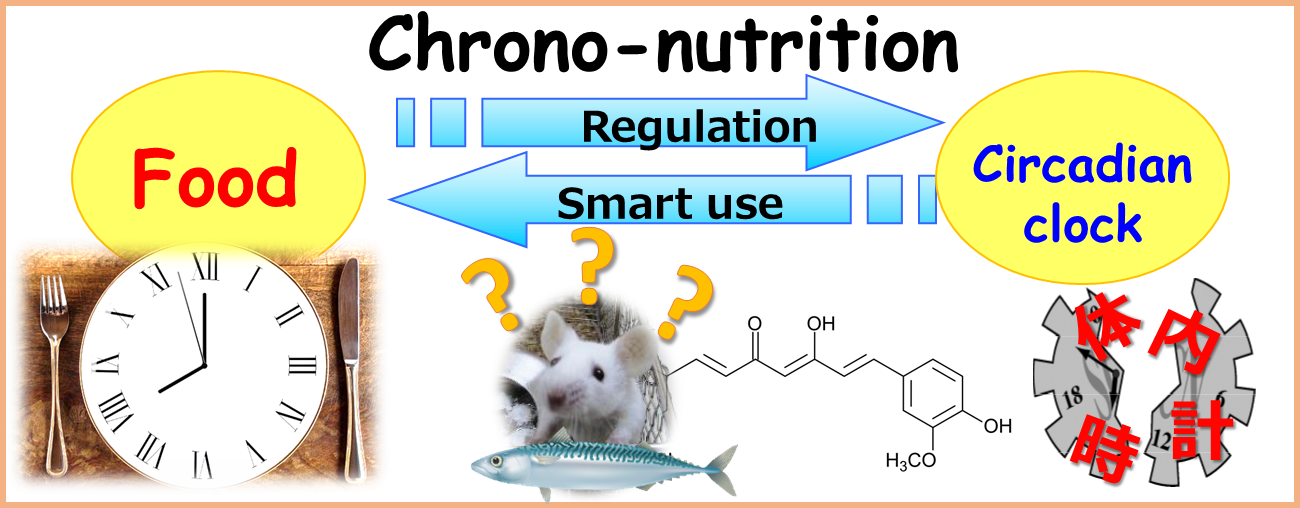
Project 2:Development of antiaging technology using chrono-nutrition
Researcher:OISHI Katsutaka
Japan is a super-aged society. In order to achieve an extension of the healthy life expectancy, it is important to prevent aging-related diseases such as sleep disorders and impaired cognition. Recent studies have revealed that circadian clock is involved in the onset of these aging-related diseases. This “Chrono-nutrition” project is focused on the development of natural substances and technologies for the prevention of sleep disorders, psychiatric diseases, impaired cognition, and metabolic diseases such as obesity and diabetes.
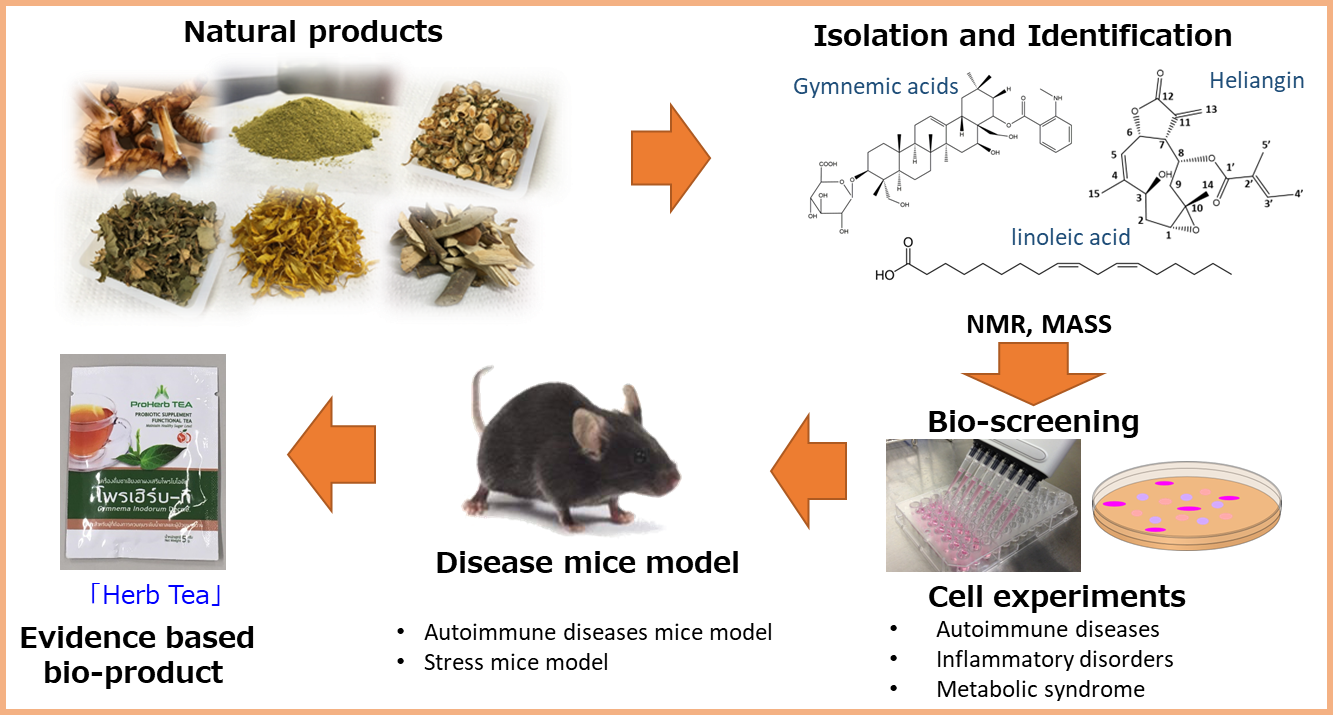
Project 3:Influence of triggered genes under progressions of depression and the related diseases
Researcher:KUWABARA Tomoko
Elucidation of the mechanisms between depression and related multiple disorders. Identification of gene expression mechanism, cell regulatory systems in multiple organs. Development of diagnostic techniques for diabetes, psychiatric and neurological disorders. Establishment of innovative strategies for diagnosis, prevention, and treatment of depression and related disorders.
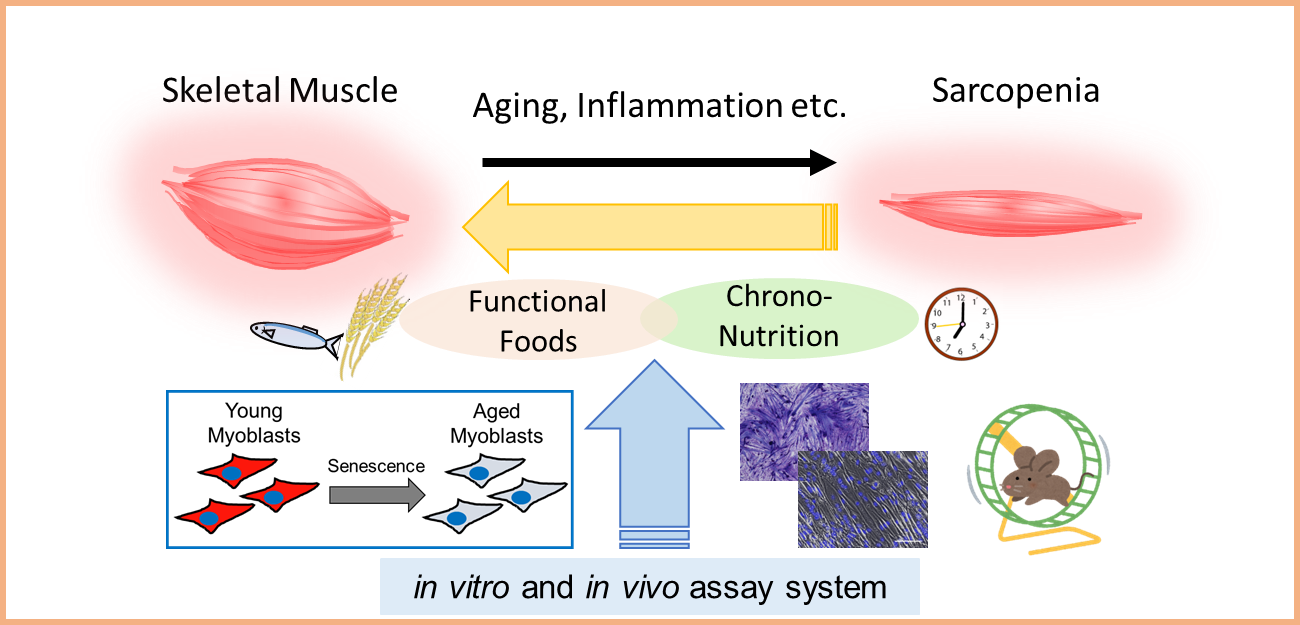
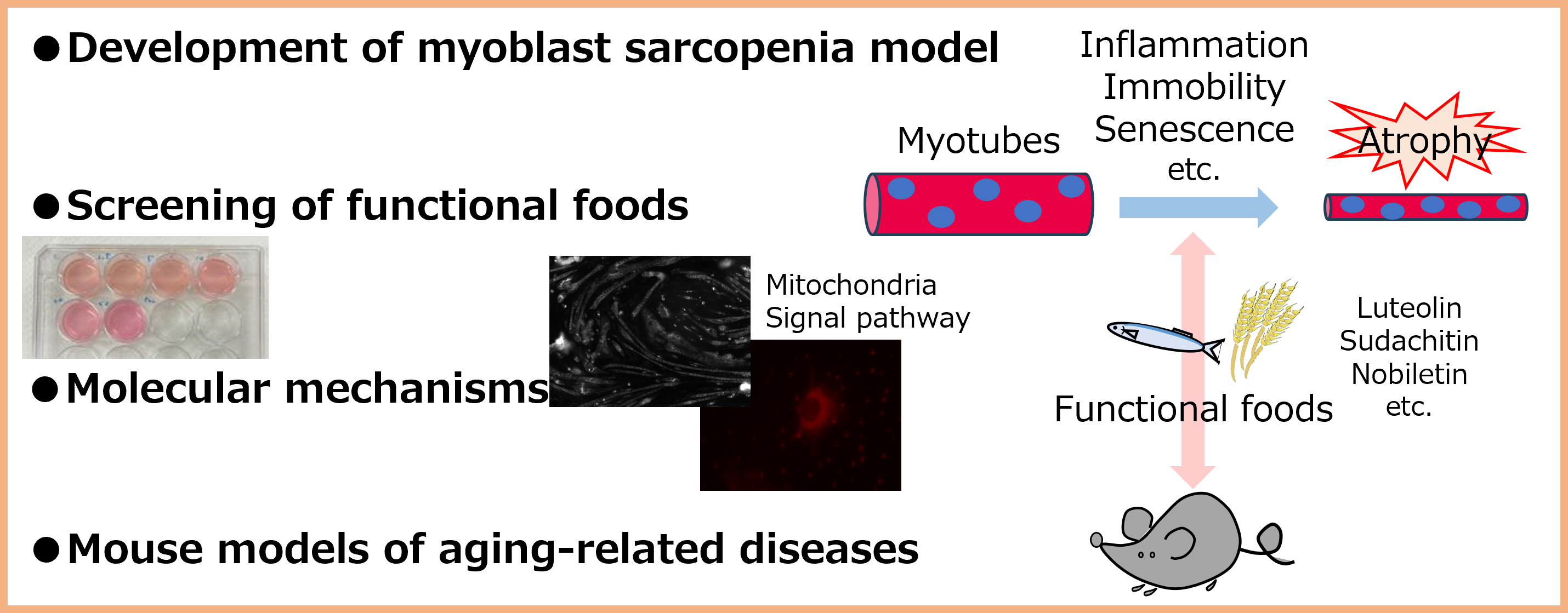
Project 4:Development of novel diet therapy for skeletal muscle health
Researcher:ABE Tomoki
Skeletal muscle comprises about 40% of body weight in human and plays important roles in regulate energy metabolism and brain function. We aim to develop a novel diet therapy to improve quality and quantity of skeletal muscle.
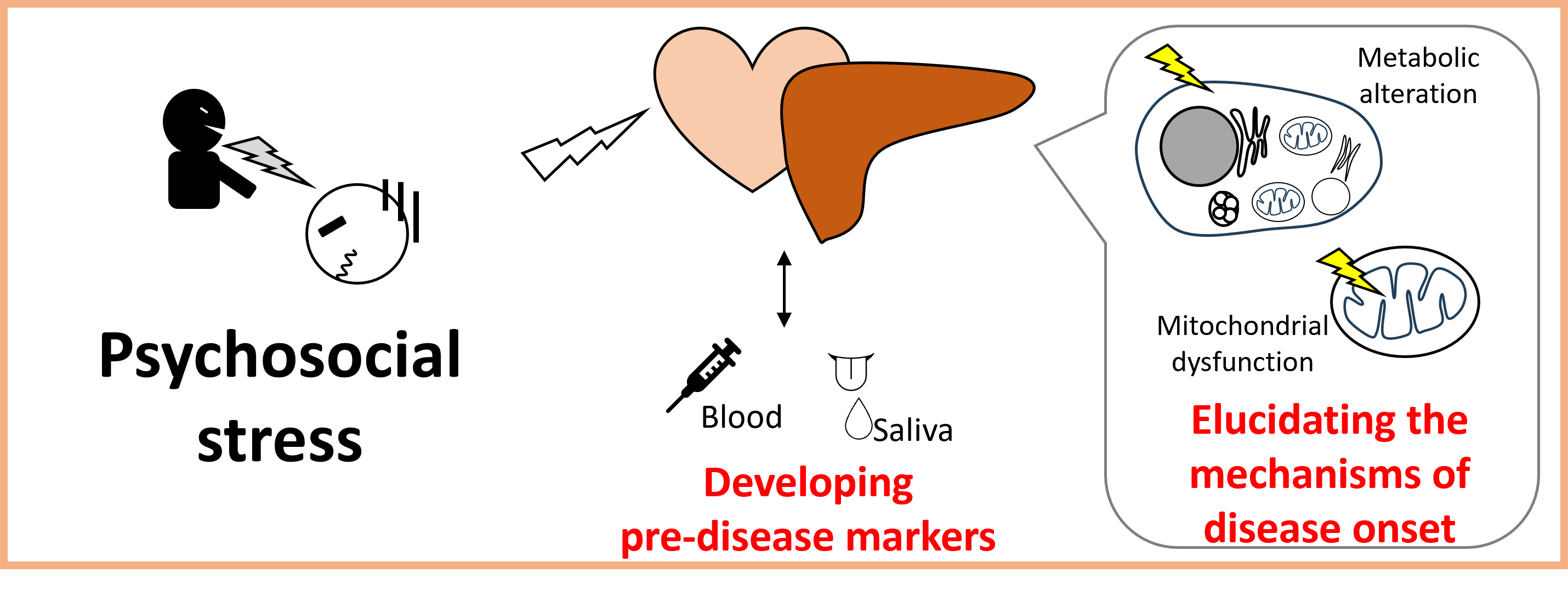
Project 5:Elucidation of the Pathogenesis of Diseases Caused by Psychological Stress and Development of Pre-disease Markers
Researcher:SATO Tomoyuki
Psychological stress is known to increase the risk of developing liver and heart diseases. This project aims to elucidate the effects of psychological stress on the liver and heart using mainly social psychological stress model mice, and to develop pre-disease markers that can predict disease onset. In addition, we are working on elucidating the mechanisms of onset of liver and neurological diseases using cultured cells and isolated mitochondria, as well as developing intervention methods based on lifestyle habits.
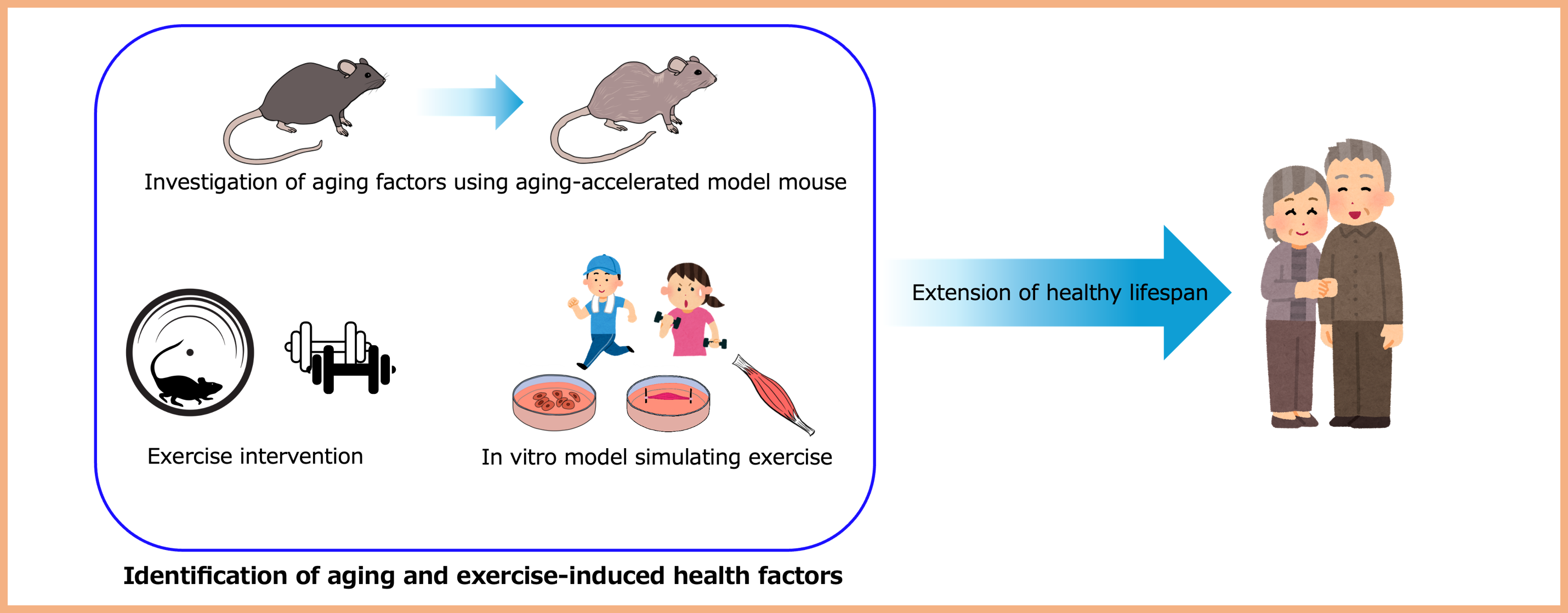
Project 6:Exploration of aging factors and mechanisms of exercise-induced pre-disease improvement
Researcher:LEE Minjung
In super-aged societies, extending healthy life expectancy is a critical issue. Aging is known to increase the risk of various diseases such as cancer and dementia. To address this, our research aims to elucidate the molecular mechanisms of aging by exploring aging-related factors using aging-accelerated model mice. Furthermore, we focus on exercise, which is believed to support health from the pre-disease stage and conduct exercise intervention experiments using laboratory animals. In parallel, we are also developing exercise-mimicking technologies at the cellular level to replicate its physiological effects. Through these studies, we aim to uncover novel molecular mechanisms involved in aging and pre-disease progression, as well as to clarify the scientific basis for the health benefits of exercise.
Member
| photo | position & name | field of expertise | and other info |
|---|---|---|---|
 |
Group reader SHINKAI Yoichi |
|
|
 |
Chief Senior Researcher OISHI Katsutaka |
|
|
 |
Senior Researcher KUWABARA Tomoko |
|
|
 |
Senior Researcher ABE Tomoki |
|
|
 |
Attached to Research Group SATO Tomoyuki |
|
|
 |
Researcher LEE Minjung |
|
|
Results
- Mishima, T; Takenaka, Y; Hashimoto-Hachiya, A; Tanigawa, Y; Suzuki, N; Oishi, K; Ogasawara, R.
Time-of-day effect of high-intensity muscle contraction on mTOR signaling and protein synthesis in mice.
SCIENTIFIC REPORTS. 2025 Jul 3;15(1):23702. doi: 10.1038/s41598-025-06709-z - Oishi, K; Yoshida, Y; Kaida, K; Terai, K; Yamamoto, H; Toyoda, A.
Potential non-invasive biomarkers of chronic sleep disorders identified by salivary metabolomic profiling among middle-aged Japanese men.
SCI REP. 2025 Apr 21;15(1):10980. doi:10.1038/s41598-025-95403-1 - Hagihara, H; Shoji, H; Hattori, S; Sala, G; Takamiya, Y; Tanaka, M; Ihara, M; Shibutani, M; Hatada, I; Hori, K;... Oishi, K; ... Miyakawa, T.
Large-scale animal model study uncovers altered brain pH and lactate levels as a transdiagnostic endophenotype of neuropsychiatric disorders involving cognitive impairment.
Elife. 2024 Mar 26:12:RP89376. doi: 10.7554/eLife.89376 - Ohkura, N; Morimoto-Kamata ,R; Kamikubo, Y; Takahashi, Y; Oishi, K.
Hypofibrinolytic phenotype in Tsumura Suzuki Obese Diabetes (TSOD) mice unrelated to hyperglycemia.
Drug Discov Ther. 2023 Nov 18;17(5):346-350. doi: 10.5582/ddt.2023.01064 - Ohkura, N; Morimoto-Kamata, R; Oishi, K; Higo-Yamamoto, S; Fujinami, A; Inoue, KI; Ohta, M.
Supplementation with Ashitaba (Angelica keiskei) yellow stem exudate prevents aging-induced thrombotic tendencies and systemic inflammation without affecting body weight gain in mice.
J Med Food. 2023 Nov;26(11):843-848. doi: 10.1089/jmf.2023.K.0140 - Oishi, K; Yajima, Y; Yoshida, Y; Hagihara, H; Miyakawa, T; Higo-Yamamoto, S; Toyoda, A.
Metabolic profiles of saliva in male mouse models of chronic sleep disorders induced by psychophysiological stress.
Sci Rep. 2023;13(1):11156. doi: 10.1038/s41598-023-38289-1 - Sato, T; Oishi, K.
Time-restricted feeding has a limited effect on hepatic lipid accumulation, inflammation and fibrosis in a choline-deficient high-fat diet-induced murine NASH model.
PLOS ONE. 2024 Jan 29;19(1):e0296950. doi: 10.1371/journal.pone.0296950 - Sato, T; Ochiishi, T; Higo-Yamamoto, S; Oishi, K.
Circadian and sleep phenotypes in a mouse model of Alzheimers disease characterized by intracellular accumulation of amyloid β oligomers.
EXP ANIM. 2023 Dec 14. doi: 10.1538/expanim.23-0104 - Zhuang, HT; Fujikura, Y, Ohkura, N; Higo-Yamamoto, S; Mishima, T; Oishi, K.
A ketogenic diet containing medium-chain triglycerides reduces REM sleep duration without significant influence on mouse circadian phenotypes.
FOOD RESEARCH INTERNATIONAL. 2023 Jul;169:112852. doi: 10.1016/j.foodres.2023.112852 - Abe, T, Sato, T, Murotomi, K.
Sudachitin and Nobiletin Stimulate Lipolysis via Activation of the cAMP/PKA/HSL Pathway in 3T3-L1 Adipocytes.
FOODS. 2023 May 10;12(10):1947. doi: 10.3390/foods12101947 - Yoshida, Y; Yajima, Y; Fujikura, Y; Zhuang, H; Higo-Yamamoto, S; Toyoda, A; Oishi, K.
Identification of salivary microRNA profiles in male mouse model of chronic sleep disorder.
Stress. 2022;26(1):21-28. doi: 10.1080/10253890.2022.2156783 - Yoshida, Y; Yajima, Y; Kawakami, K; Nakamura, S; Tsukahara, T; Oishi, K; Toyoda, A.
Salivary microRNA and metabolic profiles in a mouse model of subchronic and mild social defeat stress.
Int J Mol Sci. 2022;23:14479. doi: 10.3390/ijms232214479 - Horikawa, K; Hashimoto, C; Kikuchi, Y; Makita, M; Oishi, K.
Wheat alkylresorcinol increases fecal lipid excretion and suppresses feed efficiency in mice depending on the time of supplementation.
Nutrition. 2022;103-104:111796. doi: 10.1016/j.nut.2022.111796 - Ohkura, N; Taniguchi, M; Oishi, K; Inoue, K; Ohta, M.
Angelica keiskei (Ashitaba) has potential as an antithrombotic health food.
Food Res. 2022;6(2):18-24. doi: 10.26656/fr.2017.6(2).121 - Abe, T.
Timing of Medium-Chain Triglyceride Consumption Modulates Effects in Mice with Obesity Induced by a High-Fat High-Sucrose Diet.
NUTRIENTS. 2022 Dec 1;14(23):5096. doi: 10.3390/nu14235096 - Fujikura, Y; Yamanouchi, K; Sugihara, H; Hatakeyama, M; Abe, T; Ato, S; Oishi, K.
Ketogenic diet containing medium-chain triglyceride ameliorates transcriptome disruption in skeletal muscles of rat models of duchenne muscular dystrophy.
BIOCHEM BIOPHYS REP. 2022 Nov 11;32:101378. doi: 10.1016/j.bbrep.2022.101378 - Fujikura, Y; Kimura, K; Yamanouchi, K; Sugihara, H; Hatakeyama, M; Zhuang, H; Abe, T; Daimon, M; Morita, H; Komuro, I; Oishi, K.
A medium-chain triglyceride containing ketogenic diet exacerbates cardiomyopathy in a CRISPR/Cas9 gene-edited rat model with Duchenne muscular dystrophy.
SCI REP. 2022 Jul 8;12(1):11580. doi: 10.1038/s41598-022-15934-9 - Konishi, T; Takahashi, Y; Shiina, Y; Oike, H; Oishi, K.
Time-of-day effects of consumption of fish oil-enriched sausages on serum lipid parameters and fatty acid composition in normolipidemic adults: A randomized, double-blind, placebo-controlled, and parallel-group pilot study.
Nutrition. 2021;90:111247. doi: 10.1016/j.nut.2021.111247 - Fujikura, Y; Sugihara, H; Hatakeyama, M; Oishi, K; Yamanouchi, K.
Ketogenic diet with medium-chain triglycerides restores skeletal muscle function and pathology in a rat model of Duchenne muscular dystrophy.
FASEB J. 2021 Sep;35(9):e21861. doi: 10.1096/fj.202100629R